FDA approves Annovera ( segesterone acetate and ethinyl estradiol vaginal system )
August 10, 2018, The U.S. Food and Drug Administration today approved Annovera (segesterone acetate and ethinyl estradiol vaginal system), which is a combined hormonal contraceptive for women of reproductive age used to prevent pregnancy and is the first vaginal ring contraceptive that can be used for an entire year. Annovera is a reusable donut-shaped (ring), non-biodegradable, flexible vaginal system that is placed in the vagina for three weeks followed by one week out of the vagina, at which time women may experience a period (a withdrawal bleed). This schedule is repeated every four weeks for one year (thirteen 28-day menstrual cycles).
“The FDA is committed to supporting innovation in women’s health and today’s approval builds on available birth control options,” states Victor Crentsil, M.D., acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
Annovera is washed and stored in a compact case for the seven days not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30°C (86°F).
The efficacy and safety of Annovera were studied in three, open label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about two to four women out of 100 women may get pregnant during the first year they use Annovera.
All hormonal contraception carries serious risks. Annovera carries a boxed warning relating to cigarette smoking and serious cardiovascular events. Women over 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated and should not be used in women with:
A high risk of arterial or venous thrombotic diseases;
Current or history of breast cancer or other estrogen- or progestin-sensitive cancer;
Liver tumors, acute hepatitis, or severe (decompensated) cirrhosis;
Undiagnosed abnormal uterine bleeding;
Hypersensitivity to any of the components of Annovera; and
Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir.
The most common side effects in women using Annovera are similar to those of other combined hormonal contraceptive products and include headache/migraine, nausea/vomiting, yeast infections, abdominal pain, dysmenorrhea (painful menstruation), breast tenderness, irregular bleeding, diarrhea and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism, and the effects of CYP3A modulating drugs and tampon use on the pharmacokinetics of Annovera.
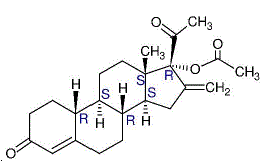
Established Name: Segesterone Acetate
Chemical Name: 16-methylene-17α-acetoxy-19-nor-pregn-4-ene-3,20-dione
Molecular Formula: C23H30O4 Molecular Weight: 370.5
Physical Form: White, or yellowish white powder
Solubility: Slightly soluble in n-hexane, soluble in ethyl acetate and methanol, freely soluble in acetone (USP classification)
Melting Point: 173 °C – 177 °C
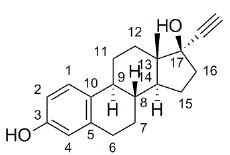
Established Name: Ethinyl Estradiol
Chemical Name: 19-Nor-17α -pregna-1,3,5(10)-trien-20-yne-3,17-diol
Molecular Formula: C20H24O2
Molecular Weight: 296.4
Physical Form: White to slightly yellowish-white crystalline powder
Solubility: Practically insoluble in water, freely soluble in alcohol, it dissolves in alkaline solution
Melting Point: 181 °C – 185 °C
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ANNOVERA is a birth control system used to prevent pregnancy in women. This system consists of a vaginal ring and two hormones.
How is this drug used?
ANNOVERA is inserted into the vagina and stays there for 3 weeks. After 3 weeks, it is removed and stays out of the vagina for one week. This schedule is repeated every 4 weeks for one year. One system (vaginal ring) is used for one year (13 cycles).
What are the benefits of this drug?
ANNOVERA prevents pregnancy, however, about 2 to 4 out of 100 women may get pregnant during the first year they use ANNOVERA.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All patients in the trials were women.
- Race: ANNOVERA prevents pregnancy in women of all races. However, a higher pregnancy rate was observed in the first year of ANNOVERA use among women who were not White.
- Age: All women in the trials were between 18 and 41 years of age; therefore, differences in response among age groups could not be determined.
The FDA granted approval of Annovera to The Population Council, Inc.
URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm616541.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm619311.htm
URL: https://www.rxlist.com/annovera-drug.htm
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

