FDA approves Braftovi ( encorafenib )
On June 27, 2018, the Food and Drug Administration approved encorafenib and binimetinib (BRAFTOVI and MEKTOVI, Array BioPharma Inc.) in combination for patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test.
Approval was based on a randomized, active-controlled, open-label, multicenter trial (COLUMBUS; NCT01909453) in 577 patients with BRAF V600E or V600K mutation-positive unresectable or metastatic melanoma. Patients were randomized (1:1:1) to receive binimetinib 45 mg twice daily plus encorafenib 450 mg once daily, encorafenib 300 mg once daily, or vemurafenib 960 mg twice daily. Treatment continued until disease progression or unacceptable toxicity.
The major efficacy measure was progression-free survival (PFS) using RECIST 1.1 response criteria and assessed by blinded independent central review. The median PFS was 14.9 months for patients receiving binimetinib plus encorafenib, and 7.3 months for the vemurafenib monotherapy arm (hazard ratio 0.54, 95% CI: 0.41, 0.71, p<0.0001). Overall response rates assessed by central review were 63% and 40%, respectively. Median response duration was 16.6 months vs. 12.3 months, respectively.
The most common (≥25%) adverse reactions in patients receiving the combination were fatigue, nausea, diarrhea, vomiting, abdominal pain, and arthralgia. Discontinuation of therapy due to adverse reactions occurred in 5% of patients receiving the combination; the most common reasons were hemorrhage and headache.
FDA today also granted approval of the THxID BRAF Kit (bioMérieux) as a companion diagnostic for these therapeutics.
The recommended doses are binimetinib 45 mg orally twice daily and encorafenib 450 mg orally once daily.
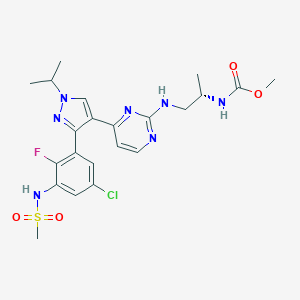
| Name: | Braftovi |
|---|---|
| CAS No.: | 1269440-17-6 |
| Formula: | C22H27ClFN7O4S |
| Chemical Names: | LGX818; LGX-818; Encorafenib (LGX818); UNII-8L7891MRB6 |
| Molecular Weight: | 540.011 g/mol |
The FDA approved the combination of Braftiovi and Mektovi based on the results of the Phase III clinical trial COLUMBUS. Compared to vemurafenib alone, Braftiovi+Mektovi doubled the median progression-free survival (mPFS) (7.3 months vs 14.9 months). FDA approves Mektovi ( binimetinib )
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BRAFTOVI is a drug which is to be used with another drug, binimetinib, to treat a type of skin cancer called melanoma. BRAFTOVI is only to be used in patients who have melanoma with a specific abnormal gene (called a BRAF V600E or V600K mutation) that has spread to other parts of the body (advanced melanoma) or cannot be removed by surgery.
How is this drug used?
BRAFTOVI is a tablet. A total of 450 mg is taken by mouth once daily with binimetinib.
What are the benefits of this drug?
BRAFTOVI plus binimetinib delays disease worsening. In addition, 63% of patients taking BRAFTOVI plus binimetinib experienced complete or partial shrinkage of their tumors.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: BRAFTOVI worked similarly in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how well the drug worked among races could not be determined.
- Age: BRAFTOVI worked similarly in patients younger and older than 65 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm611981.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm613272.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/50922675
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

