FDA approves Daurismo ( glasdegib )
November 21, 2018, The U.S. Food and Drug Administration today approved Daurismo (glasdegib) tablets to be used in combination with low-dose cytarabine (LDAC), a type of chemotherapy, for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adults who are 75 years of age or older or who have other chronic health conditions or diseases (comorbidities) that may preclude the use of intensive chemotherapy.
“Intensive chemotherapy is usually used to control AML, but many adults with AML are unable to have intensive chemotherapy because of its toxicities. Today’s approval gives health care providers another tool to use in the treatment of AML patients with various, unique needs. Clinical trials showed that overall survival was improved using Daurismo in combination with LDAC compared to LDAC alone for patients who would not tolerate intensive chemotherapy,” said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
AML is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of abnormal white blood cells in the bloodstream and bone marrow. The National Cancer Institute at the National Institutes of Health estimates that in 2018, approximately 19,520 people will be diagnosed with AML and approximately 10,670 patients with AML will die of the disease. Almost half of the adults diagnosed with AML are not treated with intensive chemotherapy because of comorbidities and chemotherapy related toxicities.
The efficacy of Daurismo was studied in a randomized clinical trial in which 111 adult patients with newly diagnosed AML were treated with either Daurismo in combination with LDAC or LDAC alone. The trial measured overall survival (OS) from the date of randomization to death from any cause. Results demonstrated a significant improvement in OS in patients treated with Daurismo. The median OS was 8.3 months for patients treated with Daurismo plus LDAC compared with 4.3 months for patients treated with LDAC only.
Common side effects reported by patients receiving Daurismo in clinical trials include low red blood cell count (anemia), tiredness (fatigue), bleeding (hemorrhage), fever with low white blood cell count (febrile neutropenia), muscle pain, nausea, swelling of the arms or legs (edema), low platelet counts (thrombocytopenia), shortness of breath (dyspnea), decreased appetite, distorted taste (dysgeusia), pain or sores in the mouth or throat (mucositis), constipation and rash.
The prescribing information for Daurismo includes a Boxed Warning to advise health care professionals and patients about the risk of embryo-fetal death or severe birth defects. Daurismo should not be used during pregnancy or while breastfeeding. Pregnancy testing should be conducted in females of reproductive age prior to initiation of Daurismo treatment and effective contraception should be used during treatment and for at least 30 days after the last dose. The Boxed Warning also advises male patients of the potential risk of drug exposure through semen and to use condoms with a pregnant partner or a female partner that could become pregnant both during treatment and for at least 30 days after the last dose. Daurismo must be dispensed with a patient Medication Guide that describes important information about the drug’s uses and risks. Patients should also be advised not to donate blood or blood products during treatment. Health care providers should also monitor patients for changes in the electrical activity of the heart, called QT prolongation.
The FDA granted this application Priority Review designation. Daurismo also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
| Name: | Daurismo |
|---|---|
| CAS No.: | 1095173-27-5 |
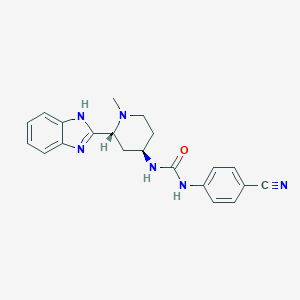
| Formula: | C21H22N6O |
| Chemical Names: | Glasdegib; PF-04449913; PF 04449913; UNII-K673DMO5H9; 1-((2R,4R)-2-(1H-benzo[d]iMidazol-2-yl)-1-Methylpiperidin-4-yl)-3-(4-cyanophenyl)urea |
| Molecular Weight: | 374.448 g/mol |
Glasdegib is a novel oral Smoothened Protein (SMO) inhibitor in combination with low-dose cytarabine (LDAC) for the treatment of AML.
AML is the most common type of malignant leukemia in adults and has the lowest survival rate of all leukemia types. According to the National Cancer Institute (NCI), approximately 19,520 patients were diagnosed with AML in 2018, and approximately 10,670 patients died. The standard treatment for AML is high-intensity chemotherapy. However, almost half of adult patients diagnosed with AML cannot receive standard high-intensity chemotherapy due to comorbidities and chemotherapy-related toxicity.
The safety and efficacy of glasdegib was assessed primarily in a clinical trial involving 111 newly diagnosed AML patients randomized to receive glasdegib in combination with LDAC or LDAC alone. The median overall survival was 8.3 months in patients receiving glasdegib plus LDAC, and the median overall survival was 4.3 months in patients receiving LDAC alone. The overall survival of the two groups differed by 4 months. , statistically significant.
Common adverse reactions to glasdegib reported by the FDA include anemia, fatigue, hemorrhage, febrile neutropenia, muscle pain, nausea, edema, thrombocytopenia, difficulty breathing, loss of appetite, indigestion, mucositis, constipation and rash. Changes in cardiac electrical activity (eg, QT prolongation) should be monitored during clinical application of the drug.
Glasdegib has been granted priority review by the FDA and has been awarded the “orphan drug”.
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DAURISMO is used to treat adults with newly-diagnosed acute myeloid leukemia (AML).
It is to be used in patients who
- are 75 years of age or older, or
- have other medical conditions that prevent the use of standard chemotherapy.
AML is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of white blood cells in the bloodstream.
How is this drug used?
DAURISMO is a tablet used once a day together with another drug (cytarabine) at low dose.
What are the benefits of this drug?
In the trial, patients treated with DAURISMO together with low dose cytarabine lived about 8.3 months in comparison to patients treated with cytarabine alone who lived about 4.3 months.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: DAURISMO worked similarly in men and women.
- Race: Most of the patients were White. Differences in how well DAURISMO worked among races could not be determined because of the small number of patients of other races.
- Age: DAURISMO worked similarly in patients younger and older than 75 years of age.
The FDA granted the approval of Daurismo to Pfizer.
URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm626443.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm628175.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/25166913
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

