FDA approves Diacomit ( stiripentol )
On August 20th, the US FDA announced the approval of Biocodex's Diacomit (stiripentol), which was used in combination with clobazam to treat seizures in patients with Dravet syndrome over 2 years of age. It is worth mentioning that this is also the 31st new drug approved by the US FDA.
Dravet syndrome is also known as Severe Myoclonic Epilepsy of infancy (SMEI). It is a rare refractory epilepsy syndrome with an incidence of approximately 1 in 15700. 80% of patients carry genetic mutations on the SCN1A gene. The age of onset of epilepsy in patients is early, and epilepsy associated with fever occurs in the first year after birth, and the frequency of episodes is high and lasts for a long time, which has a serious impact on children's development and intelligence.
The current treatment of Dravet syndrome is very limited, and patients need continuous care, which can have a serious impact on the quality of life of patients and their families. Patients with Dravet syndrome may die from epileptic seizures, epileptic seizures, or sudden deaths from seizures, with a current mortality rate of 15-20%. Patients and their parents urgently need innovative treatments to improve their quality of life.
Stiripentol is an anticonvulsant drug developed by Biocodex for the treatment of epilepsy. The chemical structure of the drug is different from other known anticonvulsants. It has been approved in the European Union, Canada and Japan for the treatment of patients with Dravet syndrome with valproate and clobar. The US FDA also granted its orphan drug qualification for the treatment of Dravet syndrome in 2008.
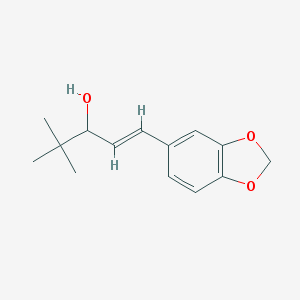
| Name: | Diacomit |
|---|---|
| CAS No.: | 49763-96-4 |
| Formula: | C14H18O3 |
| Chemical Names: | STIRIPENTOL; Diacomit; BCX 2600; Estiripentol; Stiripentolum |
| Molecular Weight: | 234.295 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DIACOMIT is a drug used along with another drug (clobazam) to treat seizures in patients with Dravet syndrome who are 2 years of age or older. Dravet syndrome is a rare and severe form of epilepsy that starts in early childhood. It is associated with difficult to control seizures and various degrees of development disability.
How is this drug used?
The total daily dose of DIACOMIT is based on a patient’s weight, and it is then divided into two or three doses. DIACOMIT can be taken as a capsule or as a powder. DIACOMIT capsules should be swallowed whole with a glass of water during a meal. DIACOMIT powder is mixed in a glass of water and should be taken right away after mixing during a meal.
What are the benefits of this drug?
Patients with Dravet syndrome taking DIACOMIT along with other seizure medications experienced fewer seizures (generalized clonic or tonic-clonic type) than patients taking placebo with other seizure medications. In Trial 1, 71% of the patients taking DIACOMIT responded to treatment compared with 5% of the placebo group. In the second trial, 67% of the patients taking DIACOMIT responded to treatment and 9% to placebo. A patient was classified as a ‘responder’ if the number of seizures in the second month of treatment was at least 50% lower than the number in the month before treatment was started.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DIACOMIT worked similarly in males and females.
- Race: Data on race were not collected per local regulatory authorities.
- Age: Age groups were too small to allow a determination of whether there were any differences among them in how well the drug works.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm618455.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/5311454
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

