FDA approves Firdapse ( amifampridine )
November 28, 2018,
The U.S. Food and Drug Administration today approved Firdapse (amifampridine) tablets for the treatment of Lambert-Eaton myasthenic syndrome (LEMS) in adults. LEMS is a rare autoimmune disorder that affects the connection between nerves and muscles and causes weakness and other symptoms in affected patients. This is the first FDA approval of a treatment for LEMS.
“There has been a long-standing need for a treatment for this rare disorder,” said Billy Dunn, M.D., director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “Patients with LEMS have significant weakness and fatigue that can often cause great difficulties with daily activities.”
In people with LEMS, the body’s own immune system attacks the neuromuscular junction (the connection between nerves and muscles) and disrupts the ability of nerve cells to send signals to muscle cells. LEMS may be associated with other autoimmune diseases, but more commonly occurs in patients with cancer such as small cell lung cancer, where its onset precedes or coincides with the diagnosis of cancer. The prevalence of LEMS is estimated to be three per million individuals worldwide.
The efficacy of Firdapse was studied in two clinical trials that together included 64 adult patients who received Firdapse or placebo. The studies measured the Quantitative Myasthenia Gravis score (a 13-item physician-rated categorical scale assessing muscle weakness) and the Subject Global Impression (a seven-point scale on which patients rated their overall impression of the effects of the study treatment on their physical well-being). For both measures, the patients receiving Firdapse experienced a greater benefit than those on placebo.
The most common side effects experienced by patients in the clinical trials were burning or prickling sensation (paresthesia), upper respiratory tract infection, abdominal pain, nausea, diarrhea, headache, elevated liver enzymes, back pain, hypertension and muscle spasms. Seizures have been observed in patients without a history of seizures. Patients should inform their health care provider immediately if they have signs of hypersensitivity reactions such as rash, hives, itching, fever, swelling or trouble breathing.
The FDA granted this application Priority Review and Breakthrough Therapy designations. Firdapse also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
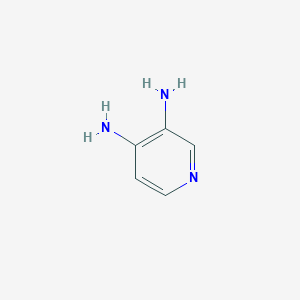
| Name: | Firdapse |
|---|---|
| CAS No.: | 54-96-6 |
| Formula: | C5H7N3 |
| Chemical Names: | 3,4-DIAMINOPYRIDINE; Pyridine-3,4-diamine; Amifampridine; 3,4-Pyridinediamine; Firdapse |
| Molecular Weight: | 109.132 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
FIRDAPSE is a drug for the treatment of Lambert-Eaton myasthenic syndrome (LEMS) in adults. LEMS is a rare disease in which the immune system attacks the connection between nerves and muscles, causing muscle weakness. Sometimes, LEMS arises as a result of cancer elsewhere in the body (condition known as paraneoplastic syndrome).
How is this drug used?
FIRDAPSE is a tablet that is taken three to four times a day by mouth.
What are the benefits of this drug?
Patients taking FIRDAPSE experienced less muscle weakness and had improved physical well-being in comparison to patients who were treated with placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: There were more women than men in FIRDAPSE clinical trials. Differences in response to FIRDAPSE between men and women could not be determined because of the small number of patients.
- Race: Most patients in the clinical trials were White. Differences in response to FIRDAPSE among races could not be determined because of the small number of patients of other races.
- Age: Most patients in the clinical trials were younger than 65 years of age. Differences in response to FIRDAPSE between patients below and above 65 years of age could not be determined.
The FDA granted the approval of Firdapse to Catalyst Pharmaceuticals, Inc.
URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm627093.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm628629.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/5918
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

