FDA approves Galafold ( migalastat )
August 10, 2018, The U.S. Food and Drug Administration today approved Galafold (migalastat), the first oral medication for the treatment of adults with Fabry disease. The drug is indicated for adults with Fabry disease who have a genetic mutation determined to be responsive (“amenable”) to treatment with Galafold based on laboratory data. Fabry disease is a rare and serious genetic disease that results from buildup of a type of fat called globotriaosylceramide (GL-3) in blood vessels, the kidneys, the heart, the nerves and other organs.
“Thus far, treatment of Fabry disease has involved replacing the missing enzyme that causes the particular type of fat buildup in this disease. Galafold differs from enzyme replacement in that it increases the activity of the body’s deficient enzyme,” said Julie Beitz, M.D., director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
Fabry disease is an inherited disorder caused by mutations (alterations) in the alpha-galactosidase A (GLA) gene located on the X-chromosome. Fabry disease is rare and affects both males and females. It is estimated that classic Fabry disease (the most severe type) affects approximately one in 40,000 males. The later-onset type is more frequent, and in some populations, may occur in one in 1,500 to 4,000 males. Patients with Fabry disease develop slowly progressive kidney disease, cardiac hypertrophy (enlargement of the heart), arrhythmias (abnormal heart rhythm), stroke and early death.
The efficacy of Galafold was demonstrated in a six-month, placebo-controlled clinical trial in 45 adults with Fabry disease. In this trial, patients treated with Galafold over six months had a greater reduction in globotriaosylceramide (GL-3) in blood vessels of the kidneys (as measured in kidney biopsy samples) as compared to patients on placebo.The safety of Galafold was studied in four clinical trials which included a total of 139 patients with Fabry disease.
The most common adverse drug reactions in patients taking Galafold in clinical trials were headache, nasal and throat irritation (nasopharyngitis), urinary tract infection, nausea, and fever (pyrexia).
Galafold was approved using the Accelerated Approval pathway, under which the FDA may approve drugs for serious conditions where there is an unmet medical need and where a drug is shown to have certain effects that are reasonably likely to predict a clinical benefit to patients. A further study is required to verify and describe the clinical benefits of Galafold, and the sponsor will be conducting a confirmatory clinical trial of Galafold in adults with Fabry disease.
Galafold was granted Priority Review designation, under which the FDA’s goal is to take action on an application within six months of application filing where the agency determines that the drug, if approved, would provide a significant improvement in treating, diagnosing or preventing a serious condition over available therapies. Galafold also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
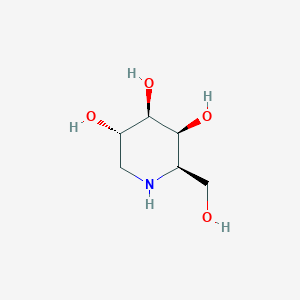
| Name: | Galafold |
|---|---|
| CAS No.: | 108147-54-2 |
| Formula: | C6H13NO4 |
| Chemical Names: | Migalastat; 1- deoxygalactonojirimycin ; DDIG; 1,5-Dideoxy-1,5- iminogalactitol ; UNII-C4XNY919FW |
| Molecular Weight: | 163.173 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
GALAFOLD is a drug for treatment of Fabry disease in adults. It is used in patients who have a specific change in the gene that causes Fabry disease. Change in the gene causes the corresponding enzyme to be a good target for therapy with GALAFOLD based on laboratory data.
Fabry disease is a rare, inherited disease that results from buildup of fat deposits called globotriaosylceramide (GL-3) in the body’s cells (including blood vessels and kidney) because of decreased activity of an enzyme called alpha galactosidase A (GLA).
How is this drug used?
GALAFOLD is taken as a capsule by mouth once every other day.
What are the benefits of this drug?
After six months of treatment, patients treated with GALAFOLD had a greater decrease in fat deposits in blood vessels of the kidney when compared to patients on placebo.
GALAFOLD was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug for serious condition while the company continues to conduct clinical trials to confirm that the drug works well.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: GALAFOLD appeared to work better in males than in females.
- Race: The majority of patients in the trial was White. Differences in how the drug works among races could not be determined.
- Age: The majority of patients in the trial was 18-65 years of age. Differences in how the drug works among different age groups could not be determined.
The FDA granted approval of Galafold to Amicus Therapeutics U.S., Inc.
URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm616598.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm619686.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/176077
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

