FDA approves Lorbrena ( lorlatinib )
On November 2, 2018, the US Food and Drug Administration approved lorlatinib (Lorbrena) to accelerate the approval of patients for the treatment of ALK-positive metastatic non-small cell lung cancer (NSCLC), which have been used in one or more types of ALK cheese. Progression on tyrosine kinase inhibitors (TKIs).
Specifically, lolatinib is approved for the treatment of metastatic disease with xalkori and at least one additional ALK inhibitor; alectinib (Alecensa) as the first ALK inhibitor for the treatment of metastatic disease; or ceritinib ( Zykadia) is the first ALK inhibitor to treat metastatic disease.
Lorlatinib is an orally available, ATP-competitive inhibitor of the receptor tyrosine kinases, anaplastic lymphoma kinase (ALK) and C-ros oncogene 1 (Ros1), with potential antineoplastic activity. Upon administration, lorlatinib binds to and inhibits both ALK and ROS1 kinases. The kinase inhibition leads to disruption of ALK- and ROS1-mediated signaling and eventually inhibits tumor cell growth in ALK- and ROS1-overexpressing tumor cells. In addition, PF-06463922 is able to cross the blood brain barrier. ALK belongs to the insulin receptor superfamily and plays an important role in nervous system development; ALK dysregulation and gene rearrangements are associated with a series of tumors. ROS1, overexpressed in certain cancer cells, plays a key role in cell growth and survival of cancer cells. Lorlatinib has been used in trials studying the basic science and treatment of Non-small Cell Lung Cancer and anaplastic lymphoma kinase (ALK)-positive Non-Small Cell Lung Cancer (NSCLC) and ROS1-positive NSCLC. Despite initial responses from the use of various ALK inhibitors, however, it is virtually almost guaranteed that all patients with the condition in question will develop tumour progression or resistance to the current therapy in use [A40086]. Considered a third-generation ALK tyrosine kinase inhibitor (TKI) for patients with ALK-positive metastatic NSCLC, lorlatinib's most optimal place in the treatment sequence of this condition has most recently been identified with its latest approval by the US FDA in November of 2018 for the indication of treating those patients' disease which has progressed even after the use of first and second-generation TKIs like crizotinib, alectinib, or ceritinib [L4848, FDA Label]. Loratinib's ability to move past the blood-brain barrier facilitates its ability to treat progressive or worsening brain metastases as well [L4848, A40078].
| Name: | Lorbrena |
|---|---|
| CAS No.: | 1454846-35-5 |
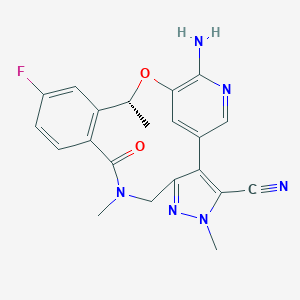
| Formula: | C21H19FN6O2 |
| Chemical Names: | Lorlatinib; PF-06463922; Loratinib; UNII-OSP71S83EU; Lorlatinib,PF-06463922 |
| Molecular Weight: |
406.421 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LORBRENA is a drug used to treat patients with a type of non-small cell lung cancer (NSCLC) that
- is caused by an abnormal anaplastic lymphoma kinase (ALK) gene and,
- has spread to other parts of your body (metastatic) and,
- has progressed while on one or more anti-cancer drugs approved for treatment of ALK-positive lung cancer.
How is this drug used?
LORBRENA is a tablet taken by mouth once a day.
What are the benefits of this drug?
Overall, 48% of patients who had measurable cancer lesions prior to taking LORBRENA had a complete or partial shrinkage of the lesions which lasted an average of 12.5 months. Approximately 60% of patients who had measurable cancer lesions in the brain prior to taking LORBRENA had a complete or partial shrinkage of the lesions in the brain which lasted an average of 19 months.
LORBRENA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: LORBRENA worked similarly among men and women.
- Race: LORBRENA worked similarly in White and Asian patients. The number of patients in other races was limited; therefore, differences among races could not be determined.
- Age: There was a limited number of patients older than 65 years of age, however, no difference in how well LORBRENA worked in patients below and above 65 years of age was observed.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm625520.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/71731823
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

