FDA approves Motegrity ( prucalopride )
Prucalopride is a dihydrobenzofurancarboxamide derivative from the benzofurane family that selectively stimulates 5-HT4 receptors and thus, it presents enterokinetic properties.[A37348] The high selectivity of prucalopride allowed further development as it prevented the cardiac adverse reactions observed due to non-target effects of precedent therapies.[A40254] Prucalopride was developed by Shire Development LLC and approved for use in Europe in 2009,[A40250] in Canada on December 7, 2011 and by the FDA on December 17, 2018.[L4880]
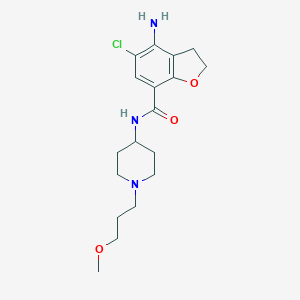
| Name: | Motegrity | |
|---|---|---|
| CAS No.: | 179474-81-8 | |
| Formula: | C18H26ClN3O3 | |
| Chemical Names: | 4-Amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide | |
| Molecular Weight: |
|
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MOTEGRITY is used to treat adults with a type of constipation called chronic idiopathic constipation (CIC).
“Idiopathic” means that the cause of the constipation is unknown.
How is this drug used?
MOTEGRITY is a tablet taken one time each day.
What are the benefits of this drug?
More patients who received MOTEGRITY experienced an increase in the number of complete spontaneous bowel movements (CSBMs) to at least 3 bowel movements per week than those who received placebo.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm629124.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/3052762
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

