FDA approves Nuzyra ( omadacycline )
Omadacycline is a new generation of aminomethylcyclotetracycline developed by Paratek Pharmaceuticals, which has a broad spectrum of activity against Gram-positive, Gram-negative and atypical bacteria.
Omadacycline has been used in trials studying the treatment of Bacterial Pneumonia, Bacterial Infections, Community-Acquired Infections, and Skin Structures and Soft Tissue Infections. Omadacycline represents a significant advance over the well-known tetracycline family, and has been shown to be highly effective in animal models at treating increasingly problematic, clinically prevalent infections caused by gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), and by gram-negative, atypical and anaerobic bacteria, including those resistant to currently available classes of antibiotics and known to cause diseases such as pneumonias, urinary tract infections, skin diseases and blood-borne infections in both the hospital and community settings.
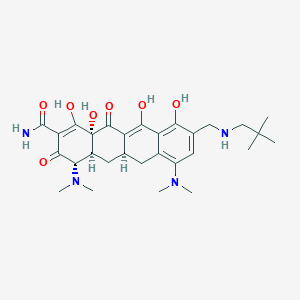
| Name: | Nuzyra |
|---|---|
| CAS No.: | 389139-89-3 |
| Formula: | C29H40N4O7 |
| Chemical Names: | Omadacycline; Amadacycline; UNII-090IP5RV8F; 090IP5RV8F; PTK-0796 |
| Molecular Weight: | 556.66 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NUZYRA is an antibacterial medicine used to treat adult patients with bacterial skin infections known as acute bacterial skin and skin structure infections (ABSSSI) caused by certain bacteria.
How is this drug used?
NUZYRA is given by a healthcare professional using a needle placed in a vein (known as intravenous or IV infusion) over 60 minutes once a day for 7 to 14 days.
NUZYRA can also be taken as a tablet by mouth once a day for 7 to 14 days.
What are the benefits of this drug?
NUZYRA worked similarly to linezolid (an antibacterial medication used to treat skin infections) in stopping the spread of ABSSSI.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: NUZYRA worked similarly in men and women.
- Race: The majority of patients was White. Differences in how well the drug worked among races could not be determined because of the small number of patients of other races.
- Age: NUZYRA worked similarly in patients younger than and older than 65 years of age.
URL: https://pubchem.ncbi.nlm.nih.gov/compound/54697325
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm624630.htm
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

