FDA approves Orilissa ( elagolix sodium )
On July 23, 2018, Abbott's key product, elagolix, was approved by the US Food and Drug Administration, which will be used to treat the pain caused by endometriosis and become the indication for more than 10 years. Come to the first new oral medication.
Elagolix will be sold under the trade name Orilissa and will be priced at $845 per month, which is an important step for Abbott as it marks the birth of another important new product. At present, Abbevi's heavy products, worth $16 billion in immunization drugs Humira will be affected by the competition of biosimilars, starting with Europe at the end of this year and new competition in the United States by 2023.
Elagolix will be available in the US early next month and is expected to grow rapidly to $1 billion in annual sales, and if the regulator approves other indications such as uterine fibroids, it will quickly exceed this figure.
| Name: | Orilissa |
|---|---|
| CAS No.: | 834153-87-6 |
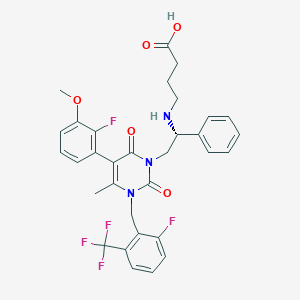
| Formula: | C32H30F5N3O5 |
| Chemical Names: | Elagolix; UNII-5B2546MB5Z; NBI-56418; 5B2546MB5Z; NBI56418 |
| Molecular Weight: | 631.6 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ORILISSA is a drug for the treatment of moderate to severe pain associated with endometriosis.
How is this drug used?
ORILISSA is a tablet and is available in two strengths. The lower strength tablet is taken once daily for no more than 24 months. The higher strength tablet is taken twice daily for no more than 6 months. Longer use is not recommended because of bone loss.
What are the benefits of this drug?
Both dose strengths of ORILISSA reduced pain during and between menstrual periods after 3 months of treatment.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All patients in the trials were women.
- Race: The majority of patients in the trials were White. Differences in how well ORILISSA worked among races could not be determined from the information available.
- Age: Patients in the trials were between 18 and 49 years of age. ORILISSA worked similarly across all age groups tested.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm616534.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/11250647
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

