FDA approves Pifeltro ( doravirine )
Doravirine has been used in trials studying the treatment of HIV-1, HIV-1 Infection, Renal Impairment, and Human Immunodeficiency Virus (HIV) Infection. In particular, doravirine is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) intended to be administered in combination with other antiretroviral medicines [FDA Label, L4562]. Doravirine is subsequently available by itself or as a combination product of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg) [L4562]. Doravirine is formally indicated for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment experience, further expanding the possibility and choice of therapeutic treatments available for managing HIV-1 infection or AIDS [L4562].
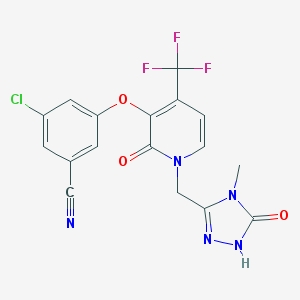
| Name: | Pifeltro |
|---|---|
| CAS No.: | 1338225-97-0 |
| Formula: | C17H11ClF3N5O3 |
| Chemical Names: | Doravirine; MK-1439; MK1439; UNII-913P6LK81M; 913P6LK81M |
| Molecular Weight: | 425.752 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PIFELTRO is a drug for the treatment of human immunodeficiency virus-1 (HIV-1) infection in adults who have not taken HIV-1 medicines before.
HIV-1 is the virus that causes acquired immune deficiency syndrome (AIDS).
How is this drug used?
PIFELTRO is a tablet that is taken by mouth once a day in combination with other drugs approved for the treatment of HIV-1 infection.
Use of PIFELTRO with some common medicines is prohibited because of interactions that affect PILFETRO’s benefit.
What are the benefits of this drug?
PIFELTRO reduced viral load of HIV-1 and is comparable to other approved drugs for treatment of HIV-1 infection.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: PIFELTRO worked similarly in men and women.
- Race: PIFELTRO worked similarly in all races.
- Age: The majority of patients in the trials were below 65 years of age. Differences in how well PIFELTRO worked between those below and above 65 years of age could not be determined.
URL: https://pubchem.ncbi.nlm.nih.gov/compound/58460047
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

