FDA approves Symdek(Tezacaftor, ivacaftor)
What is the drug for?
SYMDEKO is a drug for the treatment of cystic fibrosis (CF) in patients 12 years and older, who have specific gene mutations.
CF is a serious genetic disorder that results in the formation of thick mucus that builds up in the lungs and other parts of the body. This can lead to severe breathing problems.
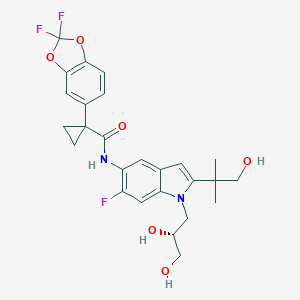
| Name: | Symdek(Tezacaftor, ivacaftor) |
|---|---|
| CAS No.: | 1152311-62-0 |
| Formula: | C26H27F3N2O6 |
| Chemical Names: | VX-661; 1152311-62-0; Tezacaftor; UNII-8RW88Y506K; VX661; VX 661 |
| Molecular Weight: | 520.505 g/mol |
How is this drug used?
SYMDEKO is packaged as two tablets. One tablet, a combination of two drugs (tezacaftor and ivacaftor) is taken by mouth in the morning. The second tablet (ivacaftor) is taken by mouth, in the evening.
What are the benefits of this drug?
SYMDEKO improved lung function by allowing air to move easier. The amount of air that can be forcibly blown out in one second [percent predicted forced expiratory volume (ppFEV1)] was increased.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: SYMDEKO worked similarly in males and females.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: SYMDEKO worked similarly in patients younger and older than 18 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm598699.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/46199646
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

