FDA approves Talzenna ( talazoparib )
Talazoparib is a once-a-day oral poly ADP ribose polymerase (PARP) inhibitor. Preclinical studies have shown that talazoparib has a strong activity and has a dual mechanism of action, which can induce tumor cell death by blocking PARP enzyme activity and capturing PARP at the site of DNA damage. Talazoparib is currently being evaluated for advanced gBRCAm breast cancer, early triple-negative breast cancer, and DDR-deficient prostate cancer, and is used in combination with various immunotherapies for the treatment of solid tumors.
Talazoparib is an orally bioavailable inhibitor of the nuclear enzyme poly(ADP-ribose) polymerase (PARP) with potential antineoplastic activity. Talazoparib selectively binds to PARP and prevents PARP-mediated DNA repair of single strand DNA breaks via the base-excision repair pathway. This enhances the accumulation of DNA strand breaks, promotes genomic instability and eventually leads to apoptosis. PARP catalyzes post-translational ADP-ribosylation of nuclear proteins that signal and recruit other proteins to repair damaged DNA and is activated by single-strand DNA breaks.
Talazoparib was approved by the FDA for use in germline BRCA mutated, HER2 negative, locally advanced or metastatic breast cancer on October 16, 2018 under the trade name Talzenna [L4661]. Talzenna was granted approval based on the results of the EMBRACA trial in which talazoparib resulted in a mean 8.6 months progression-free survival time versus physician's choice chemotherapy which resulted in 5.6 months progression-free survival.
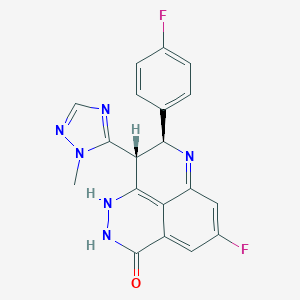
| Name: | Talzenna |
|---|---|
| CAS No.: | 1207456-01-6 |
| Formula: | C19H14F2N6O |
| Chemical Names: | Talazoparib; BMN-673; BMN 673; Talazoparib (BMN 673); UNII-9QHX048FRV |
| Molecular Weight: | 380.359 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TALZENNA is a drug for treatment of adults with a specific form of breast cancer. It is to be used in patients with:
- human epidermal growth factor receptor 2 (HER2)-negative
- an abnormal inherited BRCA gene, and
- whose cancer has spread to other parts of the body (locally advanced or metastatic).
How is this drug used?
TALZENNA capsule is taken once daily
What are the benefits of this drug?
Patients treated with TALZENNA experienced a longer time period before their tumor worsened, in comparison to patients treated with the comparator drug.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Patients were predominantly women; therefore, sex differences cannot be determined.
- Race: The majority of patients were White. The number of patients of other races was limited; therefore, differences in response among races could not be determined.
- Age: TALZENNA worked similarly in patients above and below 65 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm624335.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/44819241
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

