FDA approves Xofluza ( baloxavir marboxil )
On October 24, the US Food and Drug Administration (FDA) approved the launch of the new anti-influenza drug Xofluza (baloxavir marboxil), jointly developed by Japan’s Yanyeyi Pharmaceutical Co. and Roche, for the treatment of 12-year-old and above without complications. Acute flu patients. It is worth mentioning that this is the new anti-viral flu treatment method approved by the FDA for the first time in 20 years.
The flu is an infectious respiratory disease caused by the flu virus. Antiviral drugs can reduce symptoms and duration of illness when influenza patients are treated within 48 hours of onset.
Xofluza is an antiviral drug that is taken as a single oral dose and can be taken only once. It is capable of treating viral strains and avian influenza strains that are resistant to oseltamivir. The mechanism of action of Xofluza is different from that of existing antiviral therapies. It inhibits viral replication by inhibiting cap-dependent endonucleases in influenza viruses. The mechanism of action of anti-influenza drugs has been through targeting of neuraminidase. Compared to these drugs, Xofluza targets the earlier stages of the viral replication cycle.
Today, the U.S. Food and Drug Administration approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza (flu) in patients 12 years of age and older who have been symptomatic for no more than 48 hours.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years. With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option,” said FDA Commissioner Scott Gottlieb, M.D. “While there are several FDA-approved antiviral drugs to treat flu, they’re not a substitute for yearly vaccination. Flu season is already well underway, and the U.S. Centers for Disease Control and Prevention recommends getting vaccinated by the end of October, as seasonal flu vaccine is one of the most effective and safest ways to protect yourself, your family and your community from the flu and serious flu-related complications, which can result in hospitalizations. Yearly vaccination is the primary means of preventing and controlling flu outbreaks.”
Flu is a contagious respiratory illness caused by influenza viruses. When patients with the flu are treated within 48 hours of becoming sick, antiviral drugs can reduce symptoms and duration of the illness.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick,” said Debra Birnkrant, M.D., director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research. “Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs.”
The safety and efficacy of Xofluza, an antiviral drug taken as a single oral dose, was demonstrated in two randomized controlled clinical trials of 1,832 patients where participants were assigned to receive either Xofluza, a placebo, or another antiviral flu treatment within 48 hours of experiencing flu symptoms. In both trials, patients treated with Xofluza had a shorter time to alleviation of symptoms compared with patients who took the placebo. In the second trial, there was no difference in the time to alleviation of symptoms between subjects who received Xofluza and those who received the other flu treatment.
The most common adverse reactions in patients taking Xofluza included diarrhea and bronchitis.
Xofluza was granted Priority Review under which the FDA’s goal is to take action on an application within an expedited time frame where the agency determines that the drug, if approved, would significantly improve the safety or effectiveness of treating, diagnosing or preventing a serious condition.
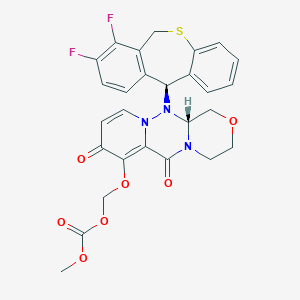
| Name: | Xofluza |
|---|---|
| CAS No.: | 1985606-14-1 |
| Formula: | C27H23F2N3O7S |
| Chemical Names: | Baloxavir marboxil; UNII-505CXM6OHG; 505CXM6OHG; Xofluza (TN); Baloxavir marboxil [INN] |
| Molecular Weight: | 571.552 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XOFLUZA is a drug to treat the flu (influenza) in people 12 years of age and older who have had flu symptoms for no more than 48 hours.
How is this drug used?
XOFLUZA is a tablet taken as a single dose by mouth within 48 hours of flu symptoms.
The dose depends on the patient’s weight.
What are the benefits of this drug?
Patients treated with XOFLUZA had a shorter time to relieve symptoms compared with patients treated with placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: XOFLUZA worked similarly in males and females.
- Race: XOFLUZA worked similarly among Whites and Asians. The number of patients in other races was limited; therefore, differences in how XOFLUZA worked among other races could not be determined.
- Age: XOFLUZA worked similarly among patients 12 to 17 years of age and those 18 years of age and older.
The FDA granted approval of Xofluza to Shionogi & Co., Ltd.
URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624226.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm624981.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/124081896
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

