FDA approves Xospata ( gilteritinib )
November 28, 2018, The U.S. Food and Drug Administration today approved Xospata (gilteritinib) tablets for the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation as detected by an FDA-approved test. The FDA also approved an expanded indication for a companion diagnostic, to include use with Xospata. The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe Technologies, Inc., is used to detect the FLT3 mutation in patients with AML.
“Approximately 25 to 30 percent of patients with AML have a mutation in the FLT3 gene. These mutations are associated with a particularly aggressive form of the disease and a higher risk of relapse,” said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “Xospata targets this gene and is the first drug to be approved that can be used alone in treating patients with AML having a FLT3 mutation who have relapsed or who don’t respond to initial treatment.”
AML is a rapidly progressing cancer that crowds out normal cells in the bone marrow and bloodstream, resulting in low numbers of normal blood cells and a continuous need for transfusions. The National Cancer Institute estimates that approximately 19,520 people will be diagnosed with AML this year; approximately 10,670 patients with AML will die of the disease in 2018.
The efficiency of Xospata was studied in a clinical trial of 138 patients with relapsed or refractory AML having a confirmed FLT3 mutation. Twenty-one percent of patients achieved complete remission (no evidence of disease and full recovery of blood counts) or complete remission with partial hematologic recovery (no evidence of disease and partial recovery of blood counts) with treatment. Of the 106 patients who required red blood cell or platelet transfusions at the start of treatment with Xospata, 31 percent became transfusion-free for at least 56 days.
Common side effects reported by patients in clinical trials were muscle and joint pain (myalgia/arthralgia), fatigue and elevated liver enzymes (liver transaminase). Health care providers are advised to monitor patients for posterior reversible encephalopathy syndrome (a syndrome characterized by headache, confusion, seizures and visual loss), prolonged QT interval (a heart rhythm condition that can potentially cause fast, chaotic heartbeats) and pancreatitis (inflammation in the pancreas). Rare cases of differentiation syndrome (symptoms of which may include fever, cough, trouble breathing, fluid around the lungs or heart, rapid weight gain, swelling, and renal or hepatic dysfunction) have been seen in patients taking Xospata. Women who are pregnant or breastfeeding should not take Xospata because it may cause harm to a developing fetus or newborn baby.
The FDA granted this application Fast Track and Priority Review designation. Xospata also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
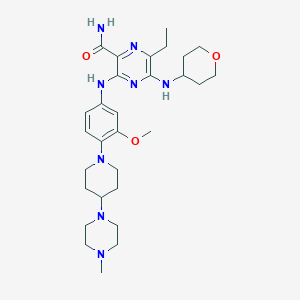
| Name: | Xospata | |
|---|---|---|
| CAS No.: | 1254053-43-4 | |
| Formula: | C29H44N8O3 | |
| Chemical Names: | Gilteritinib; ASP2215; ASP-2215; UNII-66D92MGC8M; 66D92MGC8M | |
| Molecular Weight: |
|
The drug, called XOSPATA, is approved for the treatment of relapsed or refractory acute myeloid leukemia caused by mutations in the FLT3 gene. This gene mutation exists in approximately 25% to 30% of patients with acute myeloid leukemia.
Richard Pazdur, Acting Director of the Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, US Drug Administration, said that the new drug targets the FLT3 gene and is the first approved to be used alone to treat mutations in the gene. A drug for relapsed or refractory acute myeloid leukemia.
Acute myeloid leukemia is an acute leukemia common in adults. According to the US Drug Administration, clinical trials of 138 patients with relapsed or refractory acute myeloid leukemia caused by mutations in the FLT3 gene showed that 21% of patients had complete remission (no evidence of disease and complete recovery of blood cell count) or hematology Recovery (no evidence of disease but partial recovery of blood cell count). Of the 106 patients who received red blood cell or platelet transfusion at the beginning of the trial, 31% had no need to transfuse blood for at least 56 days.
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XOSPATA is used to treat adults with acute myeloid leukemia (AML) that have a mutation in a gene called FLT3 and whose disease has come back or has not improved after previous treatment(s).
AML is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of white blood cells in the bloodstream.
How is this drug used?
XOSPATA is a tablet. Three tablets (total of 120 mg) are taken once a day.
What are the benefits of this drug?
Twenty-nine patients (21%) of the 138 patients who received XOSPATA experienced no evidence of disease and full or partial recovery of blood counts after treatment. Of the 106 patients who required red blood cell or platelet transfusions at the start of treatment with XOSPATA, 31 percent became transfusion-free for at least 56 days.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: XOSPATA worked similarly in men and women.
- Race: XOSPATA worked similarly in White and Asian patients. Differences in how well the drug worked among other races could not be determined because of the small number of patients of other races.
- Age: XOSPATA worked similarly in patients younger and older than 65 years of age.
The FDA granted the approval of Xospata to Astellas Pharma.
URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm627072.htm
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm628961.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/49803313
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

